Press Releases
Promising New OpRegen® Clinical Data Featured at 54th Annual Retina Society Meeting in Podium Presentation by Christopher D. Riemann, M.D.
Statistically Significant Evidence of a Treatment Effect with OpRegen Observed in Cohort 4 Patients
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210930005305/en/
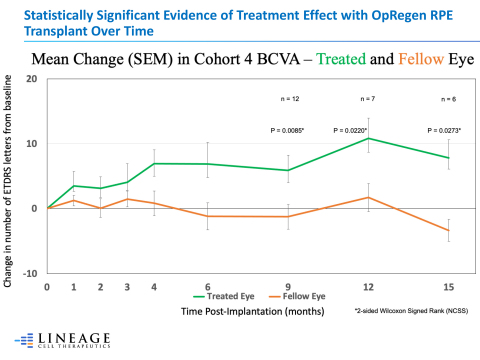
Statistically Significant Evidence of Treatment Effect with OpRegen RPE Transplant Over Time (Graphic:
Patients enrolled into the clinical study all had bilateral, advanced, atrophic AMD. OpRegen was transplanted into the subretinal space, near or across the area of geographic atrophy (GA) of their worse seeing eyes, and the patients were routinely followed as scheduled per protocol. Data presented today showed that as patients continued to progress into post-operative follow-up, eyes receiving OpRegen trended toward improvement in visual acuity, a secondary objective under the study, while their untreated eyes typically lost visual acuity, as expected with this progressive disease. As additional patients have reached longer periods post-treatment, differences in visual acuity between treated and untreated eyes across Cohort 4 patients became statistically significant beginning at month 9 (P = 0.0085), as well as months 12 (P = 0.0220) and 15 (P = 0.0273) as determined via 2-sided Wilcoxon Signed Rank (using
“This is an exciting time for the OpRegen program, the participating investigative sites, and for patients suffering from dry AMD. The efficacy findings presented today are both statistically significant as well as clinically important,” stated
“As more patients in the OpRegen trial reach clinically relevant observation periods, our data set grows larger and permits us to conduct additional analyses like the one reported today. These new data support our view that our cell transplant approach can deliver not only anatomical changes detectable by imaging studies, but also durable functional benefits to visual acuity,” stated
Dr. Riemann’s presentation will be available on the Events and Presentations section of Lineage’s website.
The
About OpRegen
OpRegen is currently being evaluated in a Phase 1/2a open-label, dose escalation safety and efficacy study of a single injection of human retinal pigment epithelium cells derived from an established pluripotent cell line and transplanted subretinally in patients with advanced dry AMD with GA. The study enrolled 24 patients into 4 cohorts. The first 3 cohorts enrolled only legally blind patients with BCVA of 20/200 or worse. The fourth cohort enrolled 12 better vision patients (BCVA from 20/65 to 20/250 with smaller mean areas of GA). Cohort 4 also included patients treated with a new “thaw-and-inject” formulation of OpRegen, which can be shipped directly to sites and used immediately upon thawing, removing the complications and logistics of having to use a dose preparation facility. The primary objective of the study is to evaluate the safety and tolerability of OpRegen as assessed by the incidence and frequency of treatment emergent adverse events. Secondary objectives are to evaluate the preliminary efficacy of OpRegen treatment by assessing the changes in ophthalmological parameters measured by various methods of primary clinical relevance. OpRegen has been well tolerated to date and there have been no new, unexpected ocular or systemic adverse events or serious adverse events that have not been previously reported. OpRegen is a registered trademark of
About Age-Related Macular Degeneration
Age-related macular degeneration (AMD) is an eye disease that can blur the sharp, central vision in patients and is the leading cause of vision loss in people over the age of 60. There are two forms of AMD: dry (atrophic) AMD and wet (neovascular) AMD. Dry (atrophic) AMD is the more common of the two forms, accounting for approximately 85-90% of all cases. In atrophic AMD, parts of the macula get thinner with age and accumulations of extracellular material between Bruch's membrane and the RPE, known as drusen, increase in number and volume, leading to a progressive loss of central vision, typically in both eyes. Global sales of the two leading wet AMD therapies were in excess of
About
Forward-Looking Statements
Lineage cautions you that all statements, other than statements of historical facts, contained in this press release, are forward-looking statements. Forward-looking statements, in some cases, can be identified by terms such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “design,” “intend,” “expect,” “could,” “can,” “plan,” “potential,” “predict,” “seek,” “should,” “would,” “contemplate,” “project,” “target,” “tend to,” or the negative version of these words and similar expressions. Such statements include, but are not limited to, statements relating to the potential benefits of treatment with OpRegen in dry AMD patients with GA, the significance of clinical data reported to date from the ongoing Phase 1/2a study of OpRegen, including the findings of retinal tissue restoration, Lineage’s plans to meet with the FDA to discuss OpRegen’s clinical development, the potential utilization of OCT imaging to measure efficacy in a pivotal clinical trial of OpRegen for the treatment of dry AMD with GA, and the potential for Lineage’s investigational allogeneic cell therapies to provide safe and effective treatment for multiple, diverse serious or life threatening conditions. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause Lineage’s actual results, performance or achievements to be materially different from future results, performance or achievements expressed or implied by the forward-looking statements in this press release, including risks and uncertainties inherent in Lineage’s business and other risks in Lineage’s filings with the
View source version on businesswire.com: https://www.businesswire.com/news/home/20210930005305/en/
(ir@lineagecell.com)
(442) 287-8963
Solebury Trout IR
(Mbiega@soleburytrout.com)
(617) 221-9660
Nic.johnson@russopartnersllc.com
David.schull@russopartnersllc.com
(212) 845-4242
Source:
